Our Science
Harnessing the regenerative power of the human body
Through a deep understanding of human biology, we are developing a pipeline of transformative small molecule therapies for the treatment of CNS disorders
-
Our Approach
-
Brain Targeting Chemistry Platform
-
Pipeline
-
ABX-002
-
ABX-003
-
ABX-101
-
Publications
01
Our Approach
Our unique approach to CNS drug development combines three distinct elements:
-
Brain-Targeting Chemistry Platform
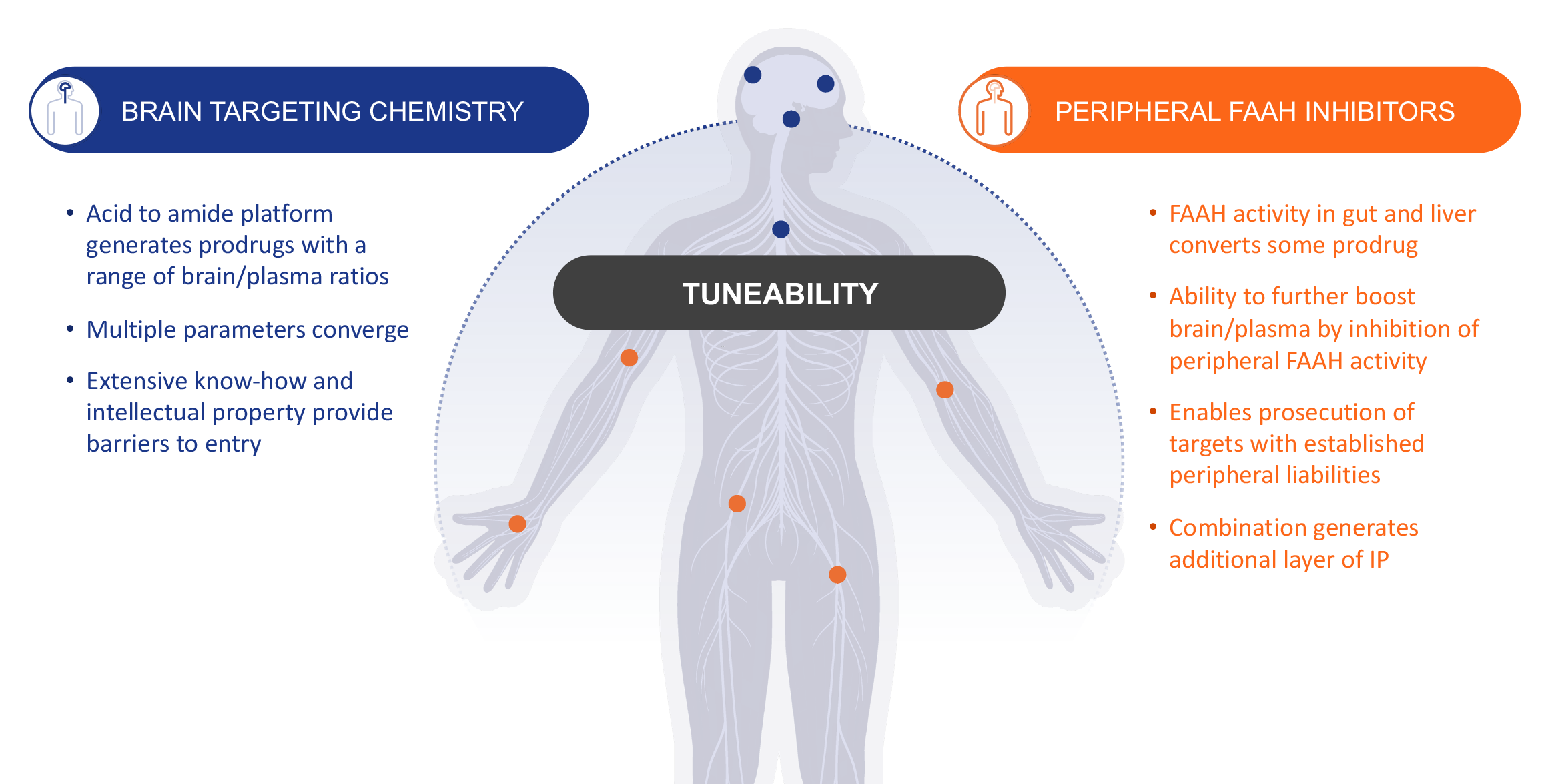
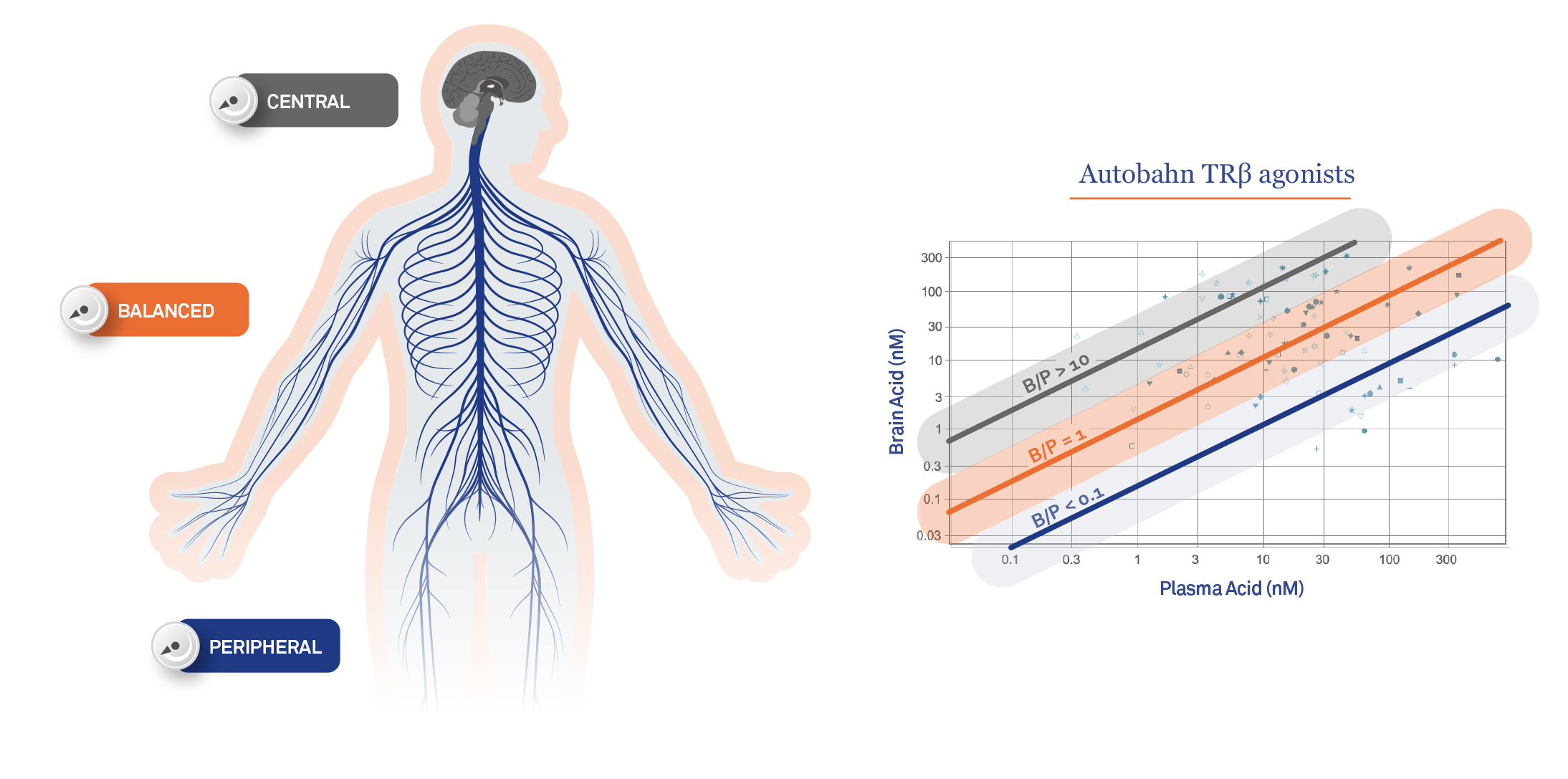
Our brain-targeting chemistry platform creates orally administered small molecule prodrugs with tunable ratios of central vs. peripheral exposure. This approach enables us to develop molecules with bespoke distribution profiles tailored to match disease pathophysiology.
-
Validated Human Biology
Our portfolio is anchored by high-confidence programs that leverage established clinical precedence, human genetics and biology, de-risking our pipeline and accelerating early development.
-
Biomarker-Driven Development
Our programs utilize biomarker-driven development strategies to demonstrate on-target / on-tissue activity and proof-of-mechanism early in the clinic.
Collectively, these elements enable us to de-risk and accelerate the development of our transformative small molecule therapies.
Brain Targeting Chemistry Platform
02
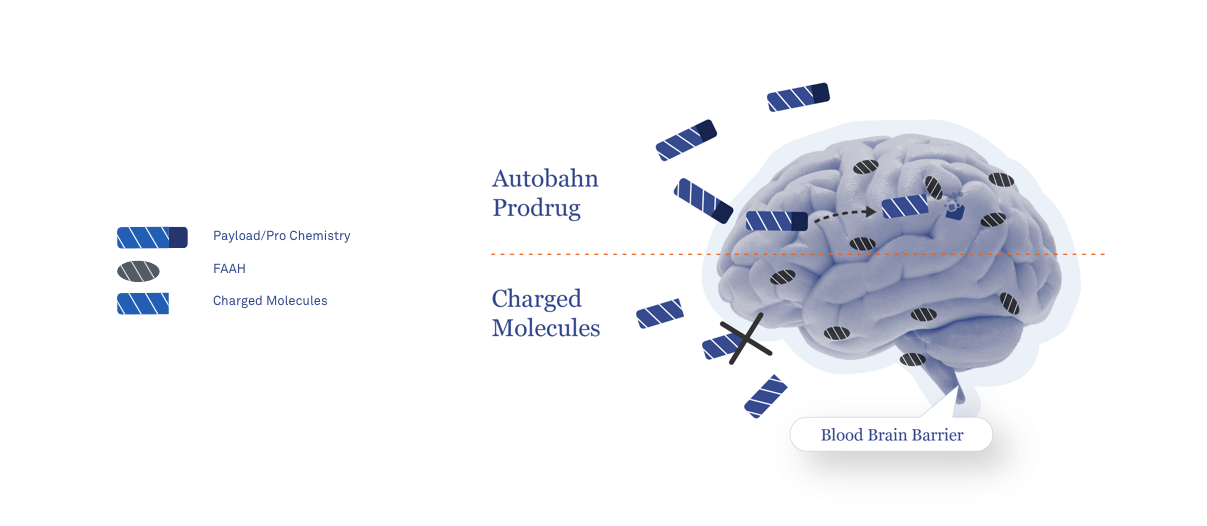
The blood-brain barrier is composed of tightly linked endothelial cells that limit the passage of pathogens and specific types of small and large molecules from the blood into the brain. This critical protective function also restricts the diffusion of therapeutics into the brain representing a major challenge to the development of new medicines for CNS diseases.
By combining the highly specialized enzymatic activity and brain-focused tissue distribution profile of fatty-acid amide hydrolase (FAAH), amplified with novel peripherally restricted FAAH inhibitors, our prodrug and peripheral restriction technology enables us to precisely tailor CNS and peripheral drug exposure.
Optimizing central versus peripheral exposure of active molecules enables us to match biodistribution with disease pathophysiology, providing Autobahn with a range of opportunities to leverage our platform to develop new therapeutics addressing unmet human health needs.
Our Pipeline
We have a pipeline of transformative small molecule therapies that are in development for the treatment of both rare and prevalent CNS disorders. Our lead molecule, ABX-002, is a potent and selective, CNS-directed thyroid hormone receptor beta (TRβ) agonist that we are developing as a potential treatment for multiple CNS disorders, initially focusing on the adjunctive treatment for people with major depressive disorder (MDD) and bipolar disorder depression.
03

04
ABX-002
MDD is the third most common cause of disability worldwide, and leaves patients suffering from an exasperated state of helplessness, grief, and increased suicidality. Further, depression remains a prominent, underserved, and chronic feature of bipolar disorder, where patients experience intense emotional states of both highs (mania or hypomania) and lows (depression). The cause of depression is not fully understood but has centered around disruption of the monoaminergic system (e.g., serotonin and norepinephrine activity). Approved drugs that enhance monoaminergic signaling in the brain have shown beneficial effects in MDD and bipolar depression, but many patients inadequately respond to current treatments, presenting the need for new treatments and novel mechanisms of actions.
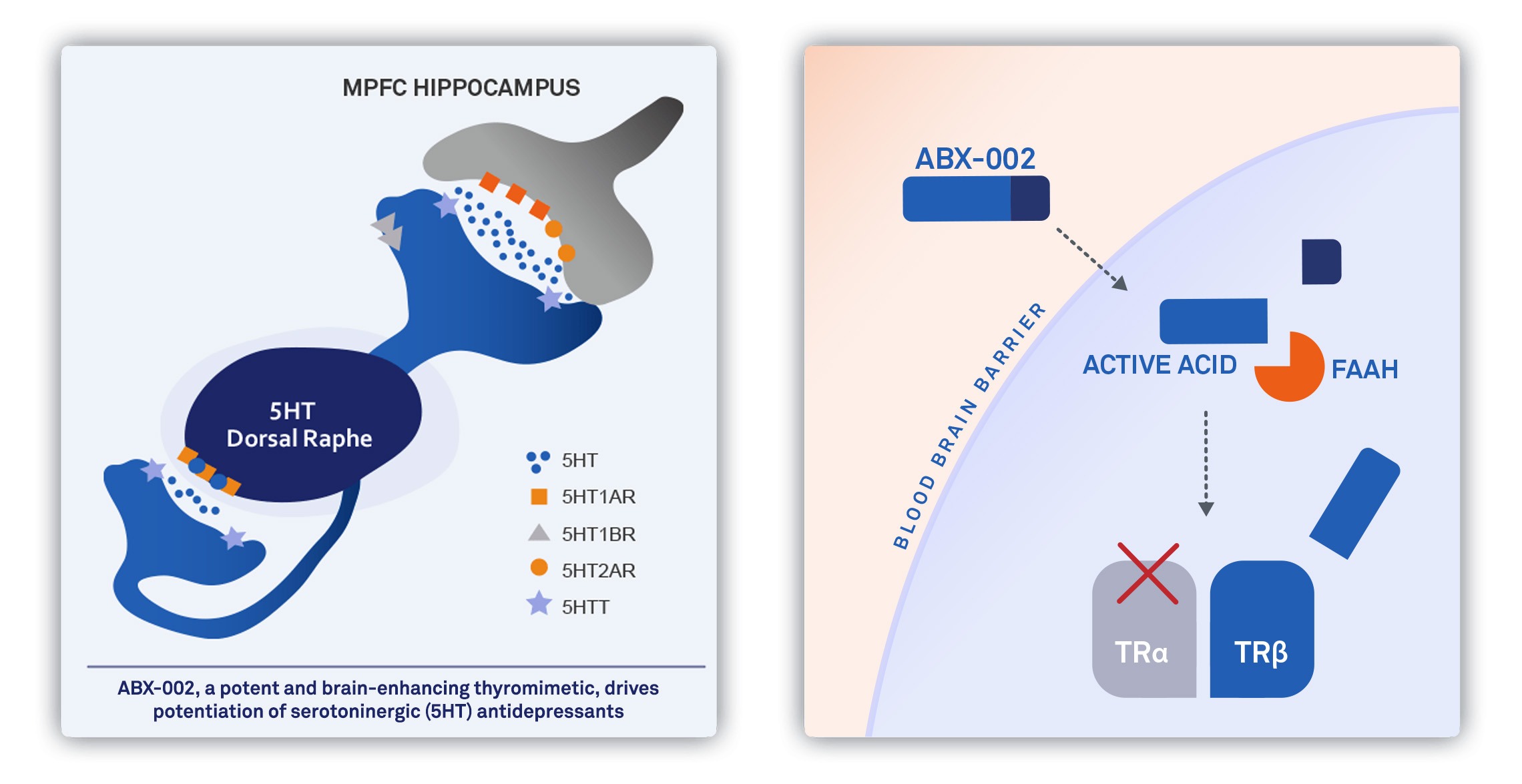
ABX-002 is a potent and selective, brain-boosting TRβ agonist that is anticipated to enhance serotonin activity and elicit a brain bioenergetic effect in the brains of people suffering from MDD. Synthetic thyroid hormone (e.g., T3 or T4) has been used off-label for over 50 years as an adjunctive treatment for MDD, with an extensive literature set and clinical use supporting its potential effectiveness as a treatment for depression. Evidence suggests that a brain-targeted thyromimetic such as ABX-002 may, in a more selective fashion, evoke favorable thyroid hormone pharmacology in the CNS, while simultaneously eliminating the peripherally-mediated side effects of synthetic thyroid hormone (i.e., cardiac effects and effects on the thyroid hormone axis).
We have completed IND-enabling activities for ABX-003, a next-generation TRβ-selective thyromimetic that utilizes combination with a peripheral FAAH inhibitor to further enhance brain distribution of free drug. ABX-003 offers a lifecycle management and franchise opportunity to maximize the value of selective and potent, CNS-active thyromimetics.
About MDD
-
Prevalence
~21M patients in the U.S. suffer from MDD
-
Patient impact
MDD patients suffer from an exasperated state of helplessness, grief, and increased suicidality
-
Current treatment
Limited safe and effective adjunctive therapies leaving patients desperate for new treatment options
05
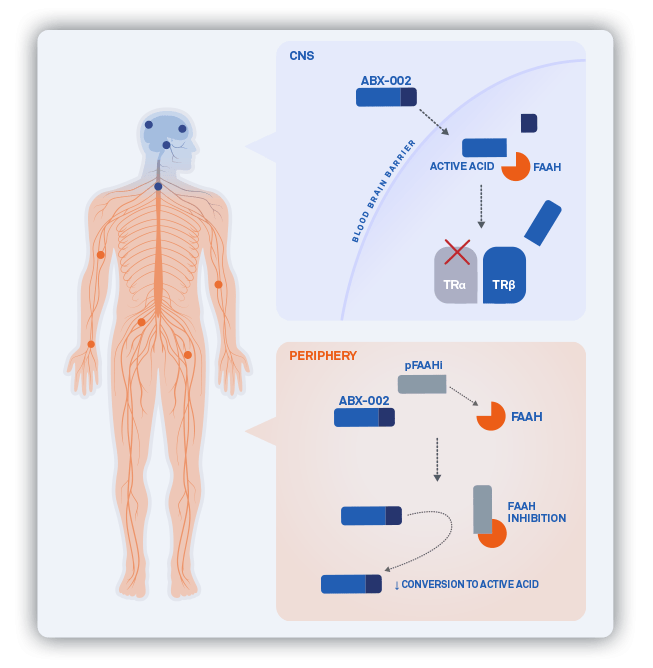
ABX-003
We have completed IND-enabling activities for ABX-003, a next-generation TRβ-selective thyromimetic that utilizes combination with a peripheral FAAH inhibitor to further enhance brain distribution of free drug. ABX-003 offers lifecycle management and franchise opportunity to maximize the value of selective and potent, CNS-active thyromimetics.
06
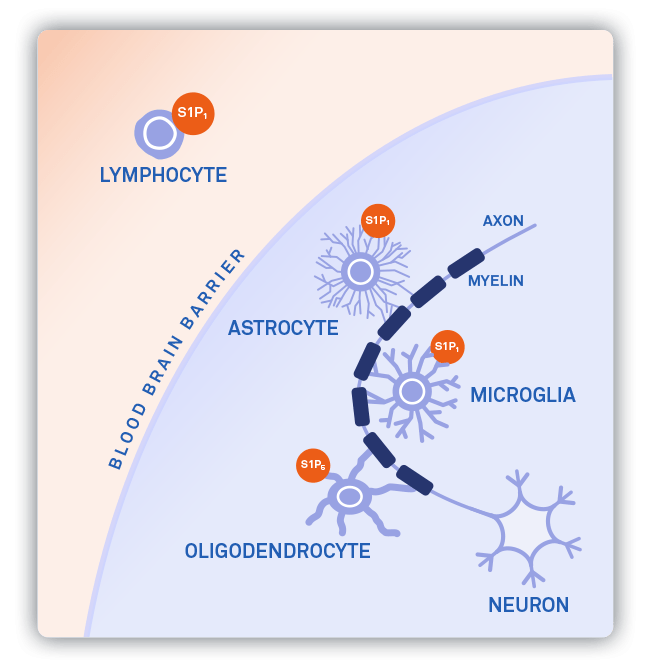
ABX-101
Sphingosine-1-Phosphate (S1P) receptor modulators are a well-studied and clinically validated class of drugs that are ideally suited for Autobahn’s FAAH-mediated brain-targeting prodrug platform. Direct neuroinflammatory and neuroprotective actions of S1P receptor modulation in the CNS are known but limited evidence exists to support CNS-specific benefit of other programs, given their inability to achieve meaningful free drug concentrations in the brain.
Autobahn is applying a rational chemistry, PK, and pharmacology approach to demonstrate improved delivery of free drug to the brain and an ability to unlock novel central pharmacology that would be applicable to both broad neuroinflammatory/neurodegenerative disorders and rare indications. Autobahn has demonstrated robust neuroinflammatory and neuroprotective benefits with ABX-101 in multiple disease-relevant nonclinical models. We are currently conducting IND-enabling studies and plan to enter the clinic in the first half of 2025.
TEST
About Autobahn
About Us